Gepotidacin for the Treatment of Uncomplicated Urogenital Gonorrhea: A Phase 2, Randomized, Dose- Ranging, Single-Oral Dose Eva
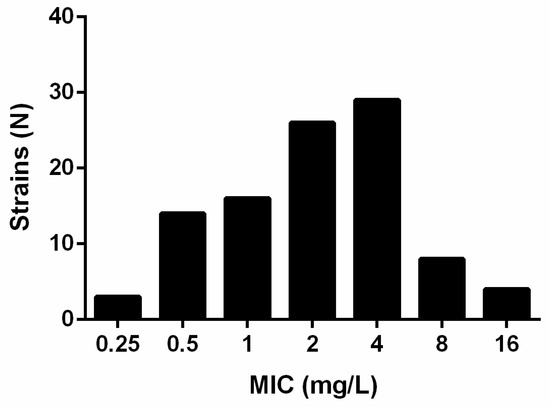
Antibiotics | Free Full-Text | Antibacterial Activity of the Novel Drug Gepotidacin against Stenotrophomonas maltophilia—An In Vitro and In Vivo Study
Design of Two Phase III, Randomized, Multicenter Studies Comparing Gepotidacin with Nitrofurantoin for the Treatment of Uncomplicated Urinary Tract Infection in Female Participants | SpringerLink

Dose Selection for Phase III Clinical Evaluation of Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | Antimicrobial Agents and Chemotherapy

Dose Selection for Phase 3 Studies Evaluating Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | GSK

Dose selection for a phase III study evaluating gepotidacin (GSK2140944) in the treatment of uncomplicated urogenital gonorrhoea | Sexually Transmitted Infections

Dose Selection for Phase 3 Studies Evaluating Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | GSK

Pharmacokinetics of Oral Formulations of Gepotidacin (GSK2140944), a Triazaacenaphthylene Bacterial Type II Topoisomerase Inhibitor, in Healthy Adult and Adolescent Participants | Antimicrobial Agents and Chemotherapy

Design of Two Phase III, Randomized, Multicenter Studies Comparing Gepotidacin with Nitrofurantoin for the Treatment of Uncomplicated Urinary Tract Infection in Female Participants | SpringerLink

Dose Selection for Phase III Clinical Evaluation of Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | Antimicrobial Agents and Chemotherapy
Antibiotics | Free Full-Text | Pharmaceutical Approaches on Antimicrobial Resistance: Prospects and Challenges

Pharmacokinetics, safety, and tolerability of gepotidacin administered as single or repeat ascending doses, in healthy adults and elderly subjects - Tiffany - 2022 - Clinical and Translational Science - Wiley Online Library