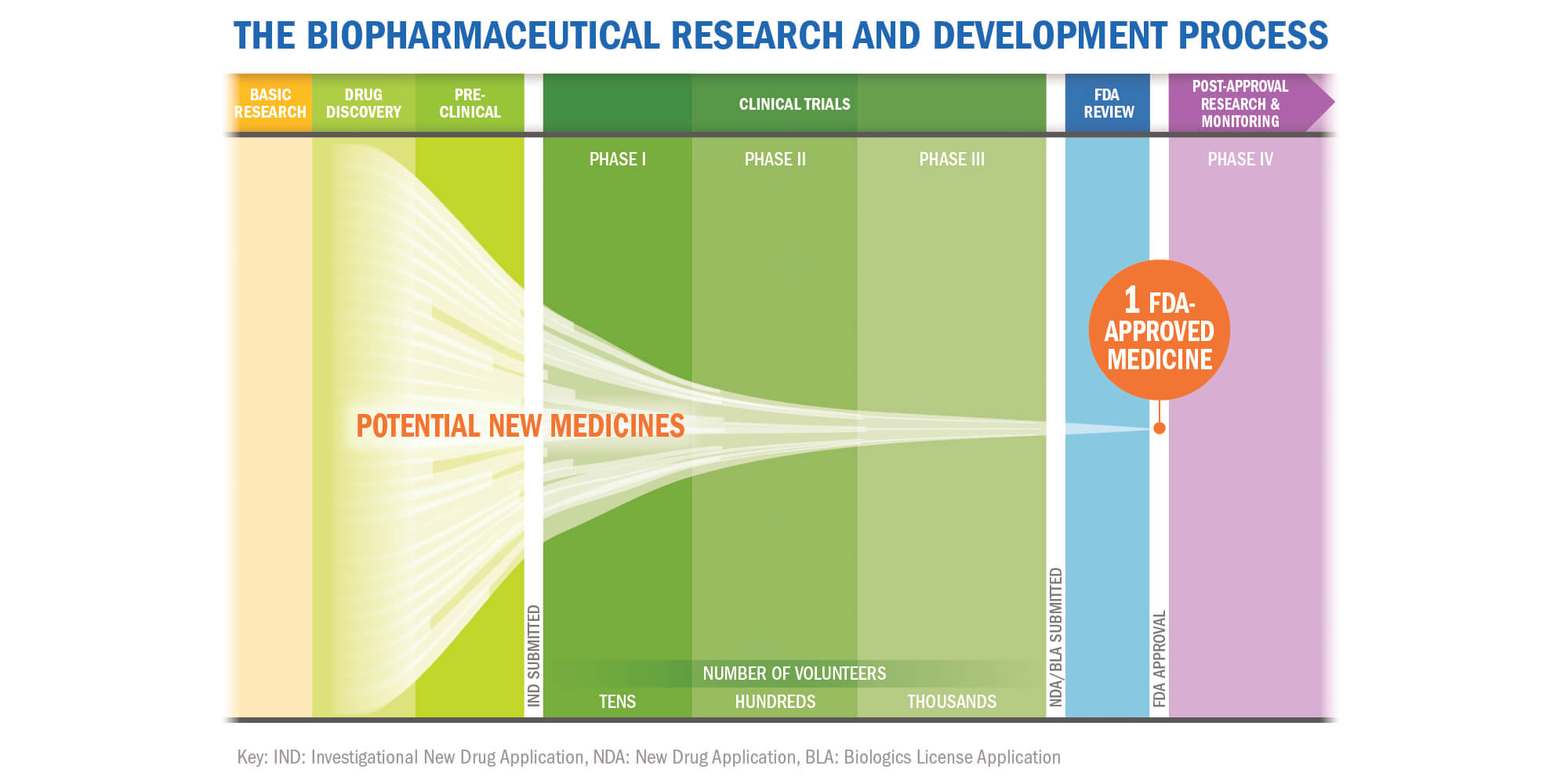
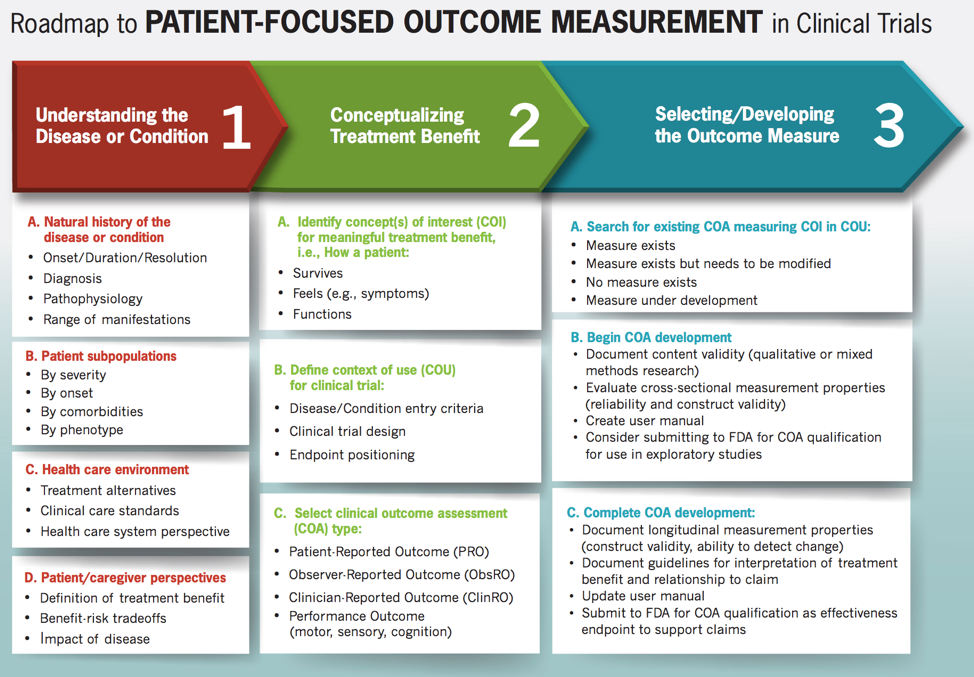
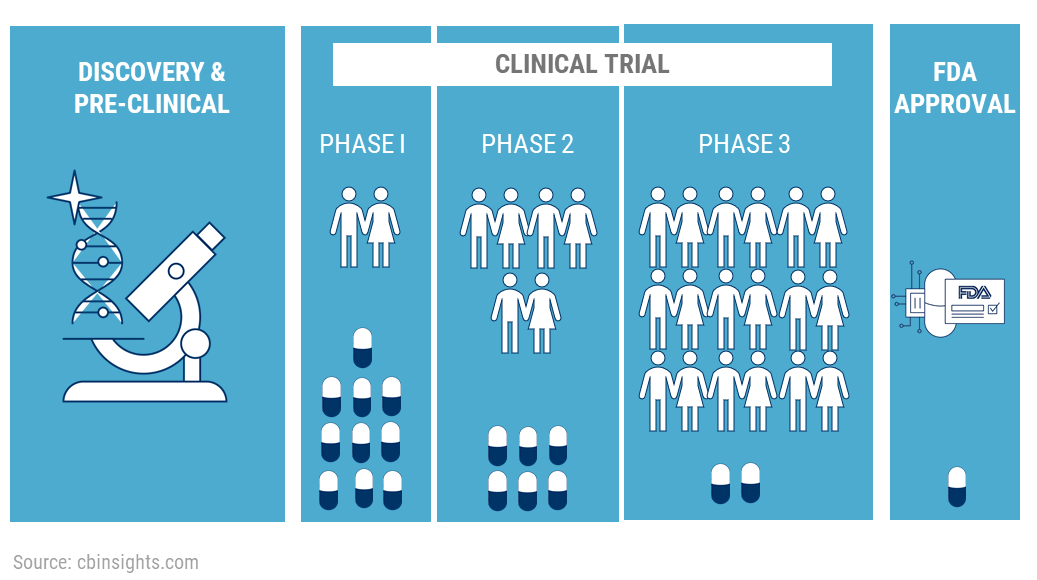
Center Watch Clinical Trials Listing; http://www.centerwatch.com/ - 1999 - Diabetes/Metabolism Research and Reviews - Wiley Online Library

Research Techniques Made Simple: Workflow for Searching Databases to Reduce Evidence Selection Bias in Systematic Reviews - ScienceDirect
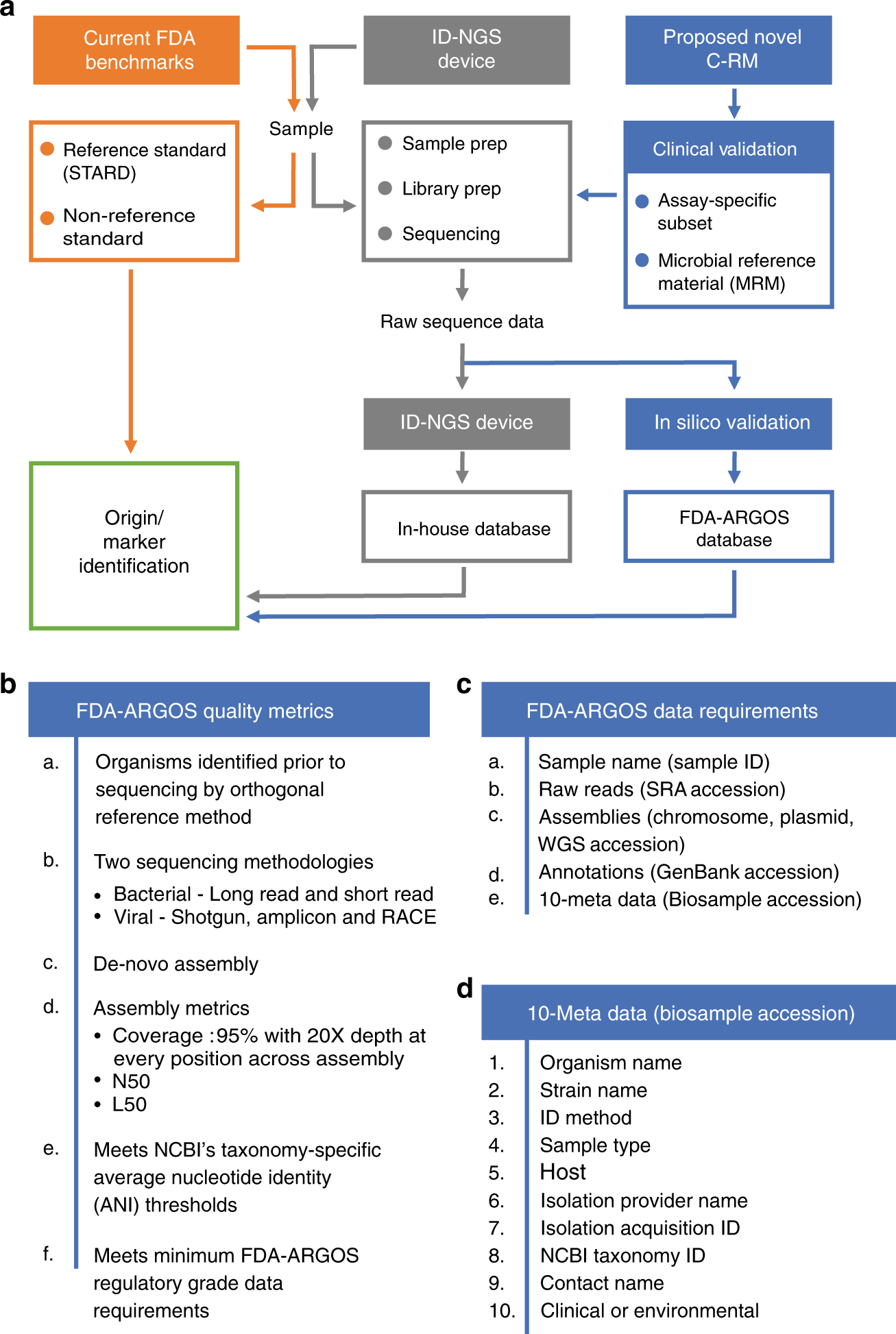
FDA-ARGOS is a database with public quality-controlled reference genomes for diagnostic use and regulatory science | Nature Communications
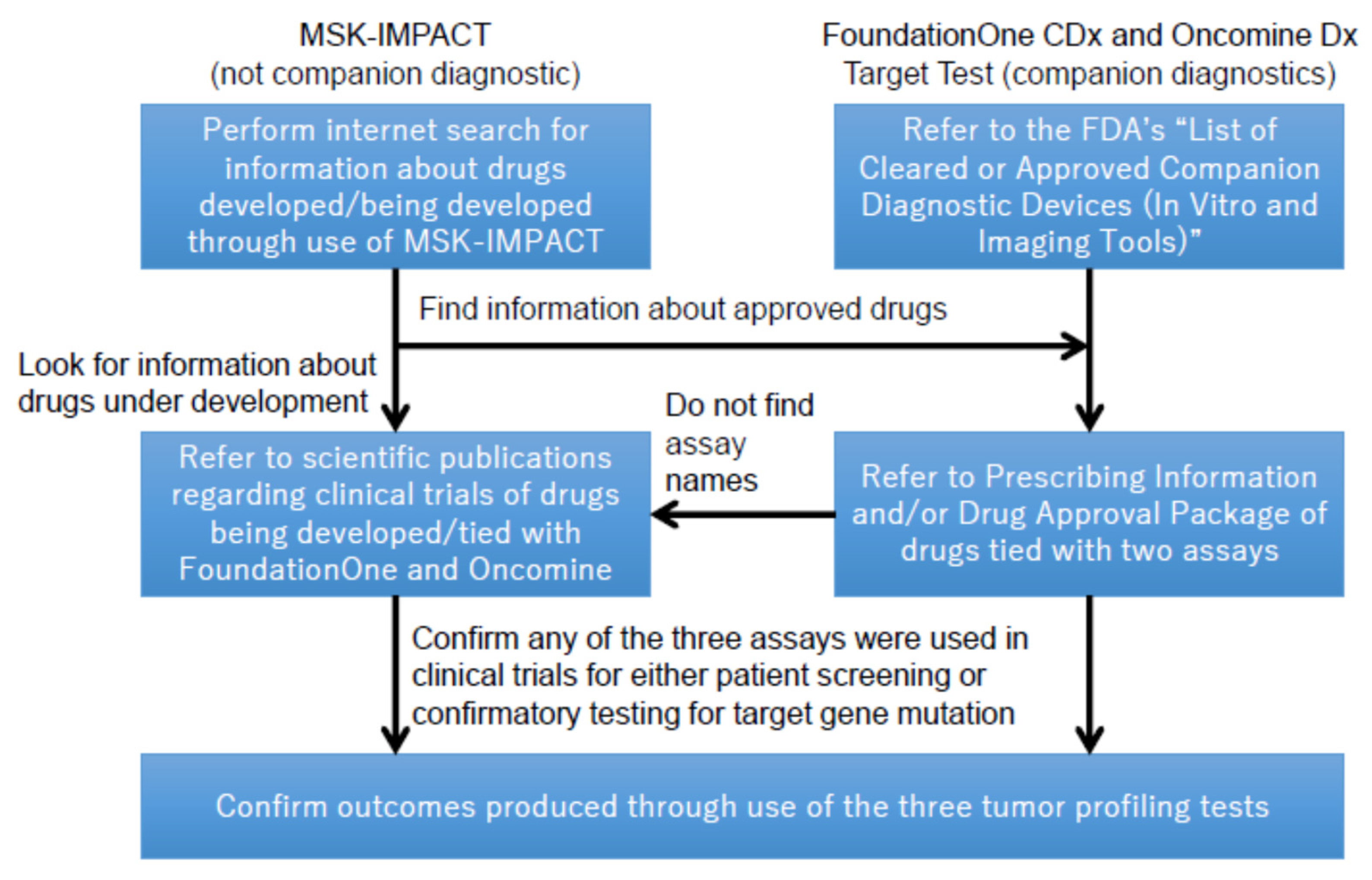
Cancers | Free Full-Text | Regulations, Open Data and Healthcare Innovation: A Case of MSK-IMPACT and Its Implications for Better Cancer Care | HTML